Washington Wedam Minyila has always associated hospital rooms with excruciating suffering. He said, “I feel like someone is sticking a knife into me.”
Wedam, 19, who has sickle cell disease, briefly allowed himself to believe what his doctors had been telling him for months: This visit might be the first step toward a cure. This was on a recent December morning. He was able to begin seeing a future free from severe pain because he was among the first people in the world to receive commercial medication for the hereditary illness.
On the day of his admission to Children’s National Hospital in Washington, D.C., for his stem cell collection—the first significant step in the ground-breaking sickle cell gene therapy process—he declared, “I choose to partially believe it.” However, that also carries with it the doubt, “Will it work?”
Since the Food and Drug Administration approved two gene therapies little over a year ago that potentially eliminate the disease’s symptoms, sickle cell patients all around the country have been asking the same question.
Only a few dozen patients around the country have had access to it since. Only a small number of hospitals have treated patients thus far due to paperwork snags, the multimillion-dollar cost, and patient worries about severe side effects.
In the US, sickle cell disease affects over 100,000 people, with 9 out of 10 being Black. Red blood cells, which are typically spherical, twist into a sickle or crescent form due to the hereditary disease. The sickle-shaped cells frequently accumulate in blood vessels, causing severe pain, strokes, organ damage, and shortened lifespans. A potential remedy is provided by the novel gene medicines from Vertex Pharmaceuticals, based in Boston, and Bluebird Bio, situated in Somerville, Massachusetts.
For this reason, Wedam overcame his doubts and persevered through months of insurance approvals and doctor’s appointments.
He said, “I know what it can mean for me,” as a group of medical experts gathered at his bedside. However, I’m not as thrilled as everyone else until it actually happens.
Sylvia, his mother, stared, holding her breath, from the corner of the room. “This is something new,” she said. And I never imagined that we would be among the first to do this, you know. It seems unbelievable.
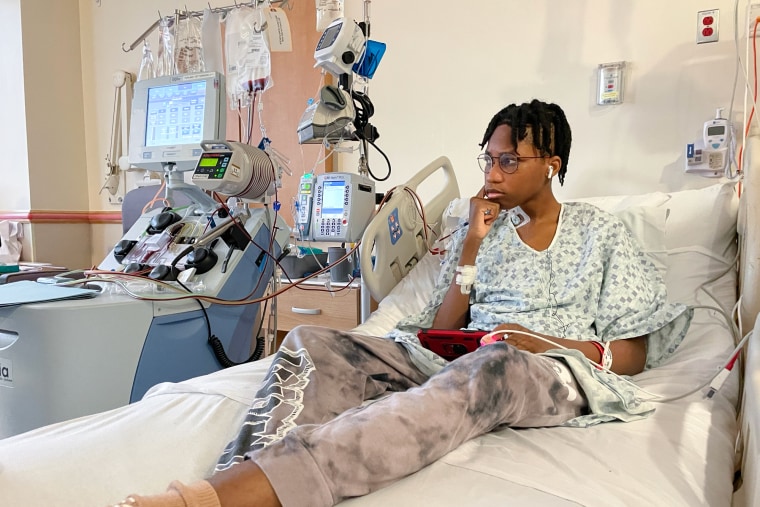
However, the first stage of what she calls her miracle was taking place right in front of her: nurses gave her son a drug that would allow stem cells that were often locked in the bone marrow to move into the bloodstream. After a few hours, Wedam was connected to a device called an apheresis machine, which extracts blood from the body and spins it rapidly to extract the millions of stem cells before reintroducing the remaining blood into the veins.
As the beginnings of the pale orange fluid carrying Wedam’s stem cells started to gather in an IV bag hanging beside him, Dr. Andrew Campell approached his bedside.
According to Campbell, director of the hospital’s Comprehensive Sickle Cell Disease Program, “you are one of the few in the country and even in the world, you know, taking this big step — gene therapy.” Since I am aware that this is still a relatively new process, it required a great deal of courage.
Wedam seems unconcerned. “I had to,” he said. There is something that I simply cannot ignore. I therefore didn’t really have an option.
A learning curve for hospitals
Wedam’s cells will be sent to a lab in Tennessee in the upcoming months, where scientists will alter a gene in the stem cells using a gene-editing method called CRISPR. This will make the red blood cells less likely to sickle and obstruct blood flow, which can cause pain crises.
In order to create space in Wedam’s bone marrow for the gene-edited cells, which a team at Children’s National Hospital will reinsert into his circulation intravenously, he will require intense chemotherapy to destroy his current stem cells.
“It’s incredible that we can provide a treatment that will cure him, keep him out of the hospital, and probably prolong his life,” said Dr. David Jacobsohn, Children’s National Blood and Marrow Transplantation Chief. What we can offer now is far more potent than it was five or ten years ago.
Jacobsohn noted, however, that not many patients had started the gene therapy treatment. Just ten individuals, including Wedam, have started or completed the process at Children’s National, which was ahead of the curve following its participation in Bluebird Bio’s clinical studies. That is one of the 1,500 sickle cell disease patients the team handles in the Washington region.
Initially, the hospital’s leadership was quite enthusiastic. We have dozens and dozens of patients that qualify, they stated. More beds must be constructed! He said, “I have a feeling that we will eventually, but it won’t happen right away.” Both the hospitals and the insurance companies have had to adjust to this new situation.
According to Jacobsohn, his team is accelerating to begin treating one or two patients each month, which he described as an exciting pace.
Keep in mind that this is an extremely risky treatment that involves the possibility of problems and significant doses of chemotherapy, he warned. Therefore, we wouldn’t want to ramp it up too quickly.
The list price of Vertex Pharmaceuticals’ months-long therapy Casgevy is $2.3 million. Lyfgenia from Bluebird Bio is valued at $3.1 million. The cost of chemotherapy or hospital stays is not covered by either.
Insurers have put in place stringent pre-authorization processes in order to control the high cost. Both pharmaceutical companies told NBC News that they have not yet received a final rejection for their products.
According to Jacobsohn, the process is resource-intensive for the few hospitals that are permitted to perform it. It necessitates several days in the hospital for the collecting of stem cells and, months later, several weeks of hospitalization for chemotherapy and the reintroduction of the stem cells. Before returning the cells to the hospital for infusion, the stem cell processing lab needs several months to genetically modify them and conduct safety inspections.
There are additional factors to take into account for patients such as Wedam. During the four days of intense chemotherapy, patients become very sick and prone to infection. Eating is practically impossible for many people who get ulcerating sores in their mouths, throats, and esophagi. Chemotherapy increases the risk of infertility and cancer over the long run.
Only one patient at the City of Hope Children’s Cancer Center in Los Angeles has finished the procedure because of all of those factors. After the early adopters begin to show results, Dr. Leo Wang, a pediatric hematologist-oncologist at the institute, says he anticipates that number to increase quickly in the upcoming months.
According to Wang, we’re really optimistic that adoption will rise and get acceptance from others who may be a little more risk adverse. We’ve already had situations when patients approach us with questions and say, “Well, I’d really like to talk to somebody who’s been through this already and get their perspective, and then maybe I’ll be interested in doing that.”
God, you did this for me
That’s one of the reasons Wedam decided to be an early adopter. “They’ll have some hope if they see me and realize that it works,” he said. even if it’s only a small amount.
He can no longer accomplish a lot of things, including getting out of bed, because of the illness. He hopes to become a filmmaker someday, nevertheless, because of the prospect of a cure.
His short-term goals are considerably more modest; he would want to take his college courses in person rather than virtually.
Now, I don t really keep up with most of my friends, so I d be able to make new ones, he said. I d be able to learn the material better if I m in person, asking the teacher actual questions. I just think, being a normal person, doing what a normal person would, I think that is what really excites me.
For nearly twenty years, the Minyila family was plagued by the illness. In addition to Wedam, his 14-year-old brother, Wekem, also has sickle cell.
It s taken almost everything, Sylvia Minyila said. It s taken our joy.
Wedam, she said, was once a happy boy who loved school and dancing. But once his pain crises started around middle school, she said, he changed.
He became withdrawn. Anywhere there was bone, he could have pain there, she said. Anywhere in the house you were, you could hear him moaning. And this is something that I used to think about regularly: Am I going to lose him? Because he was in so much pain.
Until the gene therapy, the only cure for sickle cell disease was a stem cell transplant from a donor. That worked for Wekem after Sylvia discovered she was a match for her younger son. After some scary complications, the procedure was successful.
But antibodies in Wedam s bloodstream prevented the same cure for him.
I was ecstatic. I said: God, you did this for me. But what about my other son? Sylvia said, tears rolling down her face. I wanted the same for Wedam.
She said that when the doctors at Children s Hospital called her just months later to let her know that Wedam might be a good candidate for the new gene therapy, it felt like a double miracle an answer to her prayers.
All I kept asking is: Is it curative? Is this something that he ll still have to take medications for? Is he going to have pain crises? They said, No. And I said: We re in. We are in.
Wedam has seen his younger brother transform since his treatment even using his new energy to teach himself to ride a bike. The excruciating pain before Wekem s stem cell transplant had made that impossible.
Wekem said he s looking forward to seeing his older brother be healed.
I feel like it changed the life that he is going to live, Wekem said. I didn t really think that there was going to be something else that was available to us so he could get treated and cured.
But Wedam remains skeptical. Maybe 60%, he said of his odds of being cured.
Still, even with the life-threatening complications that may come, he said, if there s any chance of a less painful future, it s worth trying.
NBC News will continue following Wedam Minyila s journey as he begins the next phases of his sickle cell treatment.
